State Of The Art (SOTA Analysis)
State of the Art (SOTA Analysis) is demonstrated by the inclusion of information related to the standard of care, current knowledge, and available treatment options in the form of devices under evaluation, similar devices, and alternatives of the medical field. During the literature search, a SOTA-specific question is framed, followed by a search using various keywords, and the PICO strategy and report are prepared accordingly.
CONTACT UF FOR GUIDANCE – FILL THE FORM
How to satisfy the requirements for SOTA Medical Devices?
By inclusion of below points in Clinical evaluation report:
- Clinical background (patient population, medical indication etc)
- Treatment alternatives available to medical conditions
- Benchmark devices
- Current status of the device/treatment and indication (current standards and applicable guidelines)
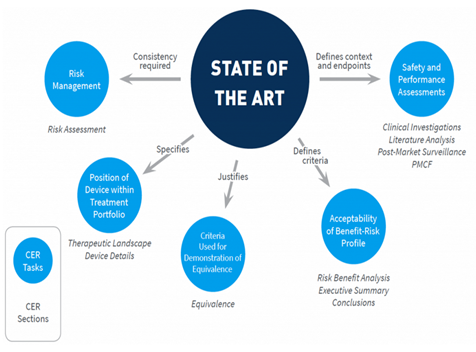
How is Clinical Evaluation Report related to State of the art (SOTA)?
According to the new medical device regulation (EU MDR), the clinical evaluation report must include the SOTA report to demonstrate the device’s appropriateness based on current knowledge or standard practice.
State of the art report preferably includes the following.
- Intended use and indication of use.
- Clinical background.
- Medical conditions.
- Epidemiological data.
- Historical aspects.
- Alternative technology available.
- Clinical practice guidelines.
- Similar devices.
- Probable complications/hazards.
- Safety and performance endpoints.
Contact us to perform literature search report writing, clinical data appraisal and analysis based on SOTA or SOA, Safety, Performance, acceptability of benefit-risk profile and undesirable side-effects, and all applicable GSPRs outlined in Annex 1 of the EU MDR article 61 and MEDDEV 2.7.1 Rev 4 which is generally accepted as best practice or benchmark for the medical condition or the treatment for which the medical device is used.
Be our Customer like 2300+ others already benefited from Timely Delivery.
Just be with us!! Be with the most Responsible service provider.


Our Social Activities